Davide Zanetti, Cristian De Luca, and Davide Bonifazi discuss the resurgence of peri-xanthenoxanthene from a forgotten dye into a versatile organic semiconductor and sustainable photocatalyst, emphasizing its importance in photoredox catalysis.
The group is led by Professor Davide Bonifazi at the Institute of Organic Chemistry in the University of Vienna.
We do research in synthetic organic chemistry to develop supramolecular architectures that, through the exploitation of their peculiar physical and structural properties, can contribute to the demonstration of key functions or basic concepts at the intersection of physical organic chemistry, materials science and biology.
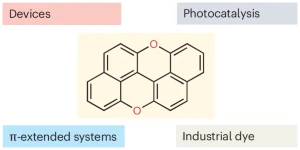
A bright comeback for peri-xanthenoxanthene

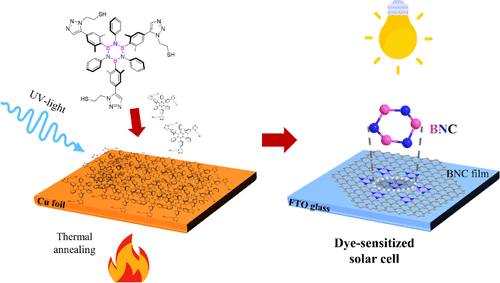
Bottom-up fabrication of BN-doped graphene electrodes from thiol-terminated borazine molecules working in solar cells

Graphene exhibits exceptional properties, including high tensile strength, mechanical stiffness, and electron mobility. Chemical functionalization of graphene with boron and nitrogen is a powerful strategy for tuning these properties for specific applications. Molecular self-assembly provides an efficient pathway for the tailored synthesis of doped graphene, depending on the molecular precursor used. This study presents a scalable approach to synthesizing large-area boron- and nitrogen-doped graphene using two borazine precursors bearing thiol functionalities. After self-assembly on electropolished polycrystalline copper foil, the precursors undergo photopolymerization under UV irradiation, and subsequent annealing in vacuum transforms the cross-linked BN-doped layer into a graphenoid structure. X-ray photoelectron spectroscopy confirms the integration of the borazine rings into the BNC architecture, while Raman spectroscopy reveals a red shift in the characteristic G bands along with intense and broad D bands, highlighting boron–nitrogen contributions. Transmission electron microscopy provides insight into the morphology and structural quality of the BNC films. The BNC films were successfully integrated as counter electrodes in dye-sensitized solar cells, achieving a power conversion efficiency of up to 6% under 1 sun illumination and 11.8% under low-intensity indoor ambient light. Hence, this work not only establishes a straightforward, controllable route for heteroatom doping but also introduces a novel concept of Pt-free counter electrodes for efficient indoor energy harvesting applications.

Catalyst-Transfer Macrocyclization Protocol: Synthesis of π-Conjugated Azaparacyclophanes Made Easy

The present Protocol describes the application of the catalyst-transfer macrocyclization (CTM) reaction, focusing on the synthesis of aza[1n]paracyclophanes (APCs). APCs are fully π-conjugated shape-persistent macrocycles with potential supramolecular chemistry and materials science applications. This method leverages the Pd-catalyzed Buchwald–Hartwig cross-coupling reaction to selectively form π-conjugated cyclic structures, a significant advancement due to its efficiency, versatility, and scalability. Overall, this Article highlights the following attributes of the CTM method: a) Efficiency and Yield: The CTM method works at mild temperatures (40 °C) and short reaction times (≥2 h), producing high yields of APCs (>75% macrocycles). It avoids the typical high-dilution conditions, making it more practical for large-scale applications. b) Versatility: The method allows the synthesis of APCs with diverse endocyclic and exocyclic functionalities and ring sizes (typically from 4- to 9-membered rings), expanding the chemical space for these compounds. This flexibility is crucial for tailoring APC properties for specific applications. c) Scalability and Reproducibility: Unlike many macrocyclization reactions, which require highly dilute conditions, CTM can perform under concentrated regimes (35–350 mM), making it more suitable for large-scale applications. d) Applications in Materials Science: APCs are noted for their potential in optoelectronic applications due to their π-conjugated structures, which are helpful in organic semiconductors, light-harvesting systems, and other advanced materials. This approach addresses the challenge of complicated multistep syntheses that have hindered the widespread integration of APCs into functional devices. A step-by-step guide to preparing exemplary APCs, including troubleshooting, is provided with photographic illustrations.
Combining broadband absorbing electrochromic materials for hybrid grey-to-colourless flexible devices
This study presents a grey-to-colourless hybrid electrochromic device combining a novel red-to-colourless polymer with blue-to-colourless Prussian blue. The device achieves a neutral tint in both the dark and bleached states, with notable changes in visible light transmittance and fast response.

Die Macht der Sonne
The Bonifazi group recently participated in the documentary Die Macht der Sonne (The Power of the Sun), which explores solar energy as an inexhaustible and sustainable source of power for the future. Click the link below to watch the documentary and learn more!


DoSChem Symposium 2024
In September, some PhD students from the Bonifazi Group took part in the annual symposium organized by DoSChem, presenting their research through posters and oral talks.
The event was a great opportunity to share our latest findings and get valuable feedback from peers. It’s always exciting to discuss our work with other researchers and hear fresh perspectives that can help shape future directions.


KinderUni 2024
Today, PhD students and postdocs from the Bonifazi Group joined the exciting KinderUni event, where curiosity and science come together for young minds!
This yearly initiative allows children to explore the wonders of science through fun, hands-on experiments. This year, our team prepared engaging activities including ball games, slime-making, and crafting bouncing balls. The children loved experimenting, learning, and discovering the science behind these creations – all while having loads of fun!
We’re thrilled to have shared our passion for science with the next generation of curious minds. Looking forward to inspiring more young scientists in future editions of KinderUni!


Lange Nacht der Forschung
Our group took part in the Lange Nacht der Forschung at the University of Vienna, a nationwide event that brings science closer to the public. For this special occasion, we prepared a series of interactive activities focused on magnetism and fluorescence, designed to engage visitors of all ages. Through hands-on experiments and live demonstrations, participants had the chance to explore fascinating physical phenomena and gain a deeper understanding of the scientific principles behind our research. It was a valuable opportunity to share our passion for science, inspire curiosity, and connect with the wider community.

Kindergarten activity

Spinoff Fellowship Demo Day
Congratulations to Laura who presented her IrrevoChrom FFG Spin-off Fellowship topic and spin-off project at the Demo Day in front of the Science Minister Martin Polaschek and the Head of the FFG Henrietta Egerth.
For more info: brutkasten.com/artikel/ffg-spin-off-fellowship-demoday

