Research Topics
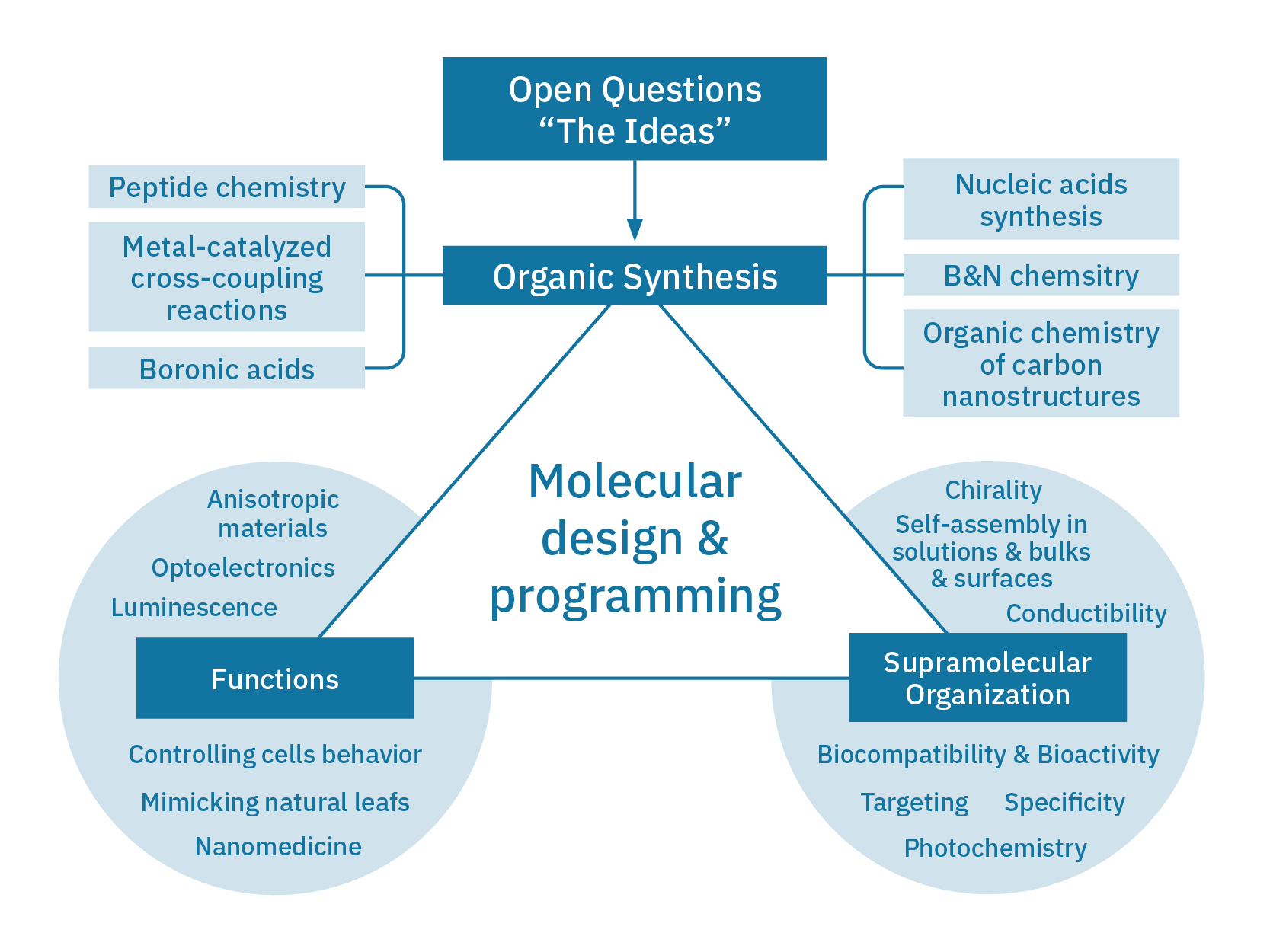
The research activities in the Bonifazi group are centred on ambitious targeted organic synthesis, carbon-based nanostructure chemistry, self-assembly and self-organisation studies to develop practical access to functional organic architectures displaying tailored properties for real world-technologies. Current research interests can be divided in four main topics:

Since its creation at the end of 2006, our team has been very active in the field of supramolecular organic chemistry at interfaces, with the objective of taking advantage of the supramolecular approach as a route to build defined two- and three-dimensional nano-patterned organic networks. We have validated the concept for which complementary H-bonding interactions can serve as powerful and versatile tool for tuning the shape of materials giving access to an enormous number of structurally-organized architectures, mastering their organization from the nanoscale up to the macroscopic level [Chem. Eur. J., 2009, 15, 7004; Coord. Chem. Rev., 2010, 254, 2342; Pure Appl. Chem., 2010, 10, 917; Nanoscale, 2013, 5, 8837]. In a collaborative research program with the group of Prof. M. Stohr (University of Groningen, Holland), exploiting the triply H-bonded uracil-2,6-diamidopyridine recognition motif linked to oligo(phenylene-ethynylene) molecular wires, we have reported the first example, at that time, of a simultaneous three-components H-bonded assembly on Ag(111) surfaces by STM analysis under ultrahigh vacuum conditions (UHV). [Angew. Chem. Int. Ed., 2008, 47, 7726]
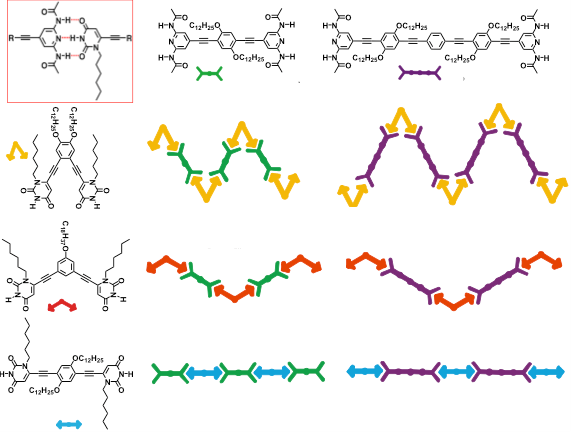
Simultaneously, for the first time, we also reported the first writable organic-based surfaces based on thermally switchable 2D porous assembly [Chem. Commun., 2009, 3525]. Discrete assemblies, featuring hosting properties towards hydrophobic porphyrin guests, have been also formed using complementary angular modules. The multicomponent non-covalent H-bonded hollowed assemblies featuring polygonal porous domains (in coll. with Prof. P Samori at Universisty of Strasbourg, France) have also been comprehensively investigated at the solid-liquid interfaces, for the first time showing that both the network and the phase depend on the molecular geometry, concentrations and the peculiar chemical structure [Chem. Commun., 2008, 5289; Adv. Funct. Mater., 2009, 19, 1207; J. Am. Chem. Soc., 2009, 131, 509; J. Am. Chem. Soc., 2009, 131, 13062]. Passing from the molecular to the nanoscale, our team has also shown how the use of H-bonding allowed the tuning of the size and shape of uniform architectures enabling a morphological change to occur from nanoparticle and vesicles [Chem. Commun., 2009, 2830]. The formation of helicoidally organized rods, crater-, prism- and crown-like morphologies have been also fully described [Chem. Eur. J., 2010, 17, 3262; Langmuir, 2011, 27, 1513].
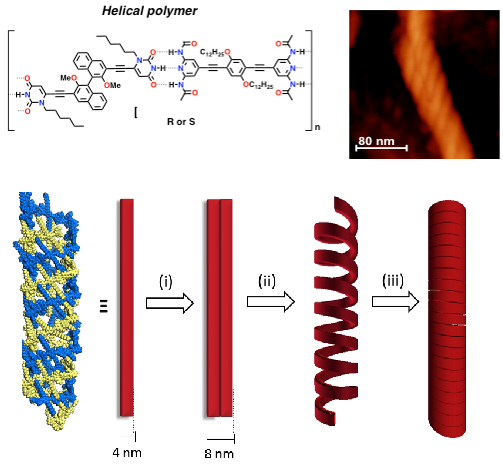
Aiming at introducing asymmetric properties and relate molecular and macroscopic chirality [ChemPlusChem, 2014, 79, 895], we have very recently engineered a new family of BINOLs derivatives undergoing solvent-dependent nanostructuration. Depending on the solvophobic properties of the liquid media, the material can be moulded into spherical, rod-like, fibrous and helical morphologies [J. Am. Chem. Soc., 2015, 137, 8150]. This behaviour is interpreted as a consequence of an interplay between the degree of association of the H-bond recognition, the vapour pressure of the solvent and the solvophobic/solvophilic character of the supramolecular adducts in the different solutions under dynamic conditions, namely during solvent evaporation conditions at room temperature.
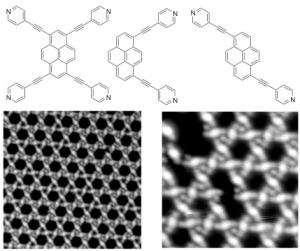
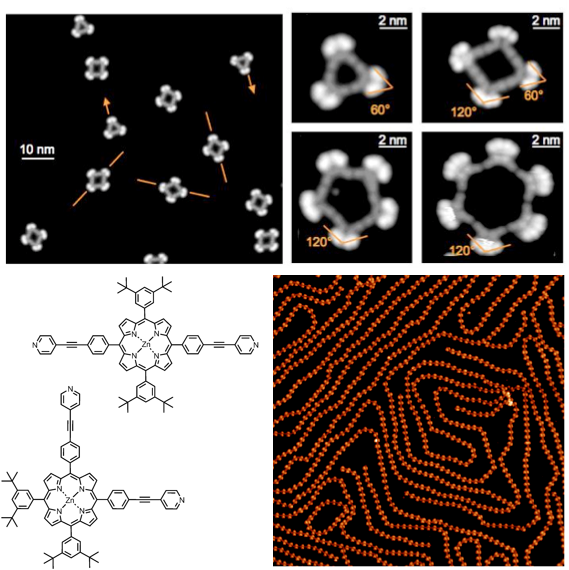
In collaboration with the group of Prof. J. Barth and Prof. W. Auwarter (TUM), we have described for the first time the formation of discrete, tunable and hollowed cyclic supramolecular architectures on insulating boron-nitrate [J. Am. Chem. Soc., 2015, 137, 2420] and on metal surfaces exploiting metal-coordination bonding interactions with pyridyl-bearing ligands [Nano Lett., 2010, 10, 122; J. Am. Chem. Soc., 2010, 132, 6783; ACS Nano, 2010, 6, 4936], the structure of which could be tailored by the geometry, conformational flexibility and functionality of the porphyrin molecular modules. Recently, we have also reported the first patterning of lanthanide and transition metal atoms on surfaces exploiting the orthogonal coordination properties of tetrapyridyl porphyrins [Angew. Chem. Int. Ed., 2015, 54, 6163]. Expanding the topic, a series of differently substituted pyridyl-bearing pyrenyl modules [ACS Nano, 2016, 10, 7665] have been prepared, all showing a different self-assembly behavior (e.g., spangling, crocheting and knitting) depending on the spatial disposition of the pyridyl groups. Extended porous networks showing inherent flexibility and adaptability properties have been prepared exploiting competing non-covalent interactions, using tripodal modules like 1,3,4-tris-pyridyl-benzene [ACS Nano, 2012, 6, 4258; Chem. Eur. J., 2013, 14143]. In a parallel study, in collaboration with the groups of Prof. G. Costantini (Warwick University, United Kingdom) and Prof. A. De Vita (King’s College, United Kingdom), we demonstrated that polyaromatic hydrocarbons form nanostructures at the interface that are ruled by an interplay between van der Waals attractions and intermolecular repulsions, the latter driven by reversible molecule-substrate charge transfer [ACS Nano, 2014, 8, 12356, Nanoscale, 2016, 8, 19004].
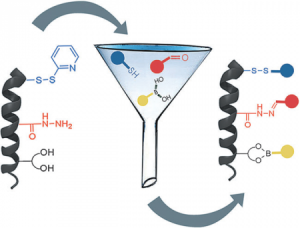
Aiming at introducing a certain functionality on porous self-assembled organic networks, we have very recently initiated a novel research project (sponsored by the ERC Starting Grant 2012, COLORLANDS) in which, taking advantage of the molecular self-assembly, we seek to create a new generation of periodically-organized organic architectures that can structurally control the arrangement of molecular chromophores and/or fluorophores. The ultimate aim is to create a library of supramolecularly-assembled architectures emitting or adsorbing light throughout the visible region that, depending on their spatial organization, are suitable for producing versatile organic materials for device implementation (e.g. an OLED with luminophores or an artificial “leafs” mimicking natural antenna if hosting chromophores). In this respect, we conceived a facile and versatile protocol for the simultaneous use of three orthogonal dynamic covalent reactions, namely disulfide, boronate and acyl hydrazine formation. Using a pre-programmed a-helix peptide, we conceived a facile and versatile protocol to spatially organize tailored blue, red and yellow chromophores [Angew. Chem. Int. Ed., 2015, 54, 15739].

The inorganic mimic of benzene, borazine, first reported by Stock in 1926 possesses a wide energy gap of ~6 eV, although displaying similarity to benzene in both geometry and in formal topology of the π-molecular orbitals. The UV emissive properties of borazine and of its potential oligomeric derivatives make such molecular substrates attractive candidates to be implemented as active layer in UV light-emitting devices (LEDs) where high photon energy is necessary for either processing or sensing/characterization purposes. Thus, we turned our attention to borazine units as substituting unit to replace aromatic six-member rings [Chem. Commun., 2015, 51, 5222]. However, the susceptibility of the BN bonds to undergo hydrolysis in the presence of moisture is one of the major deterrent toward the use of this substrate, and attempts to significantly prevent the hydrolytic decomposition of borazines by introduction of various substituents were not successful until bulky substituents were placed in the ortho positions of B-aryl groups [Chem. Eur. J., 2013, 19, 7771].
With this approach, different borazine derivatives could be prepared, laterally exposing different functional groups. The electroluminescent properties of borazine-containing LEDs and LECs devices (in coll. with Prof. F. Cacialli, UCL London, United Kingdom) showed for the first time an UV-electroluminescent behavior. One patents also cover these works [WO 2012/126842]. Exploiting the decarbonilative [4+2] cycloaddition route, we have very recently tackled the first synthesis of borazine-based dendritic structures. Upon increasing the number of the borazine cores, a dramatic enhancement the UV-centered emission quantum yield has been observed, up to 60% for the heptaborazino-doped derivative [J. Am. Chem. Soc., 2017, 139, 503]. Also, we have prepared hydroxyl- and methyl-pentaaryl borazine molecules and studied their self-assembly behavior on a metal surface to prepare unprecedented bottom-up borazine-based supramolecular architectures [Angew. Chem. Int. Ed., 2013, 52, 7410; Chem. Eur. J., 2014, 20, 11856].
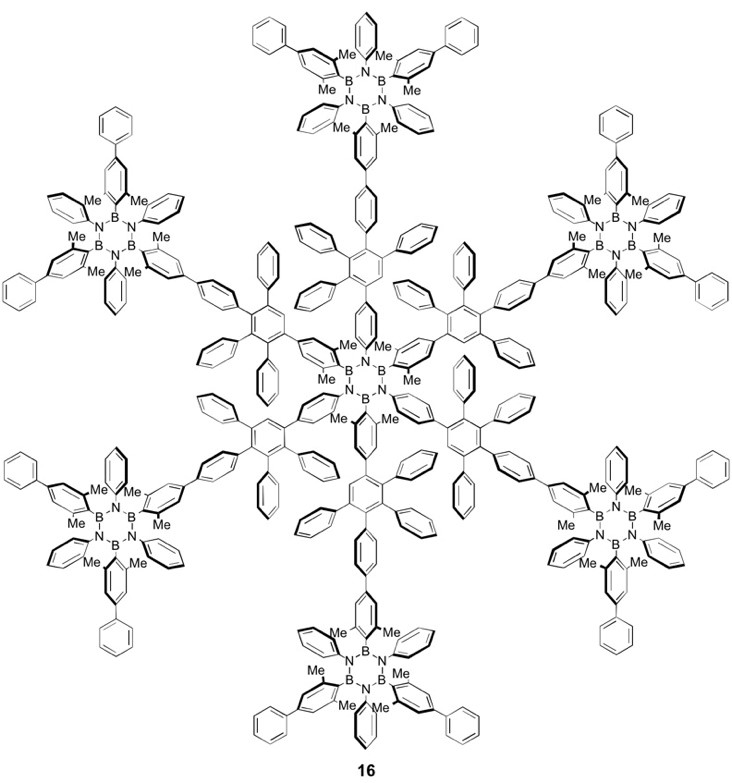
By means of the STM technique (Prof. G. Costantini, University of Warwick, United Kingdom and Prof. A. De Vita King’s College, United Kingdom) it has been found that hydroxyl-pentaaryl borazine molecules assemble in magic clusters of 7, 10, 11, 12, and 13 monomers on Cu(111). When peripheral ethynyl-phenyl substituents are added, porous networks are obtained with the borazine essentially decoupled from the metal surface (Cu or Au) [Chem. Eur. J., 2014, 20, 11856].Angew. Chem. Int. Ed., 2016, 55, 5947]. This allowed us to develop the first family of O-doped dyes, the type of O-annulation revealed to dictate the HOMO-LUMO gap and its chromatic properties.
Descending in the chalcogenic group, we also tackled the synthesis of Se- and Te-doped scaffolding, in particular the benzo-1,3-chalcogenazoles. Exceptionally, these aromatic heterocycles proved to be very stable and thus very handy to form controlled solid-state organizations in which wire-like polymeric structures are formed through secondary N…Y bonding interactions engaging the chalcogen (Y = Se or Te) and nitrogen atoms. In particular, it has been shown that the recognition properties of the chalcogen center at the solid state could be programmed by selectively barring one of its σ-holes through a combination of electronic and steric effects exerted by the substituent at the 2-position.
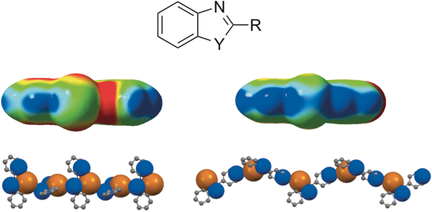
As predicted by the electrostatic potential surfaces calculated by quantum chemical modeling, the pyridyl groups revealed to be the stronger chalcogen bonding acceptors, and thus the best ligand candidate for programming the molecular organization at the solid state. The weaker chalcogen donor properties of the Se-analogues trigger the formation of feeble N…Se contacts, which are manifested in similar solid-state polymers featuring longer nitrogen-chalcogen distances [Chem. Eur. J., 2015, 21, 15377].
Cellular motility is nowadays well recognised as a key process involved in both physiological and pathological phenomena. In the immune system, an elaborate network of signalling directs leukocytes from the circulation into the surrounding tissue to destroy invading microorganisms and infected cells. In vertebrate adults, cell motility is a critical part of wound healing and tissue repair and it is also critical in disease states where the failure of migratory processes results in chronic inflammatory, vascular diseases, multiple sclerosis, and even mental retardation. Furthermore, aberrant cell migration in tumour invasion and metastasis is one the leading causes for cancer. Aiming at interfacing supramolecular chemistry with cell migration to govern its directionality, we very decided to explore this field, as it could lead to new paradigms to study new approaches for control cell growth, tumour invasion, and tissue regeneration. Physical and chemical cues such as the concentration gradients of signalling molecules are the main guiding stimuli for cell migration, inducing cell polarity and thus controlling the migration rate and direction.
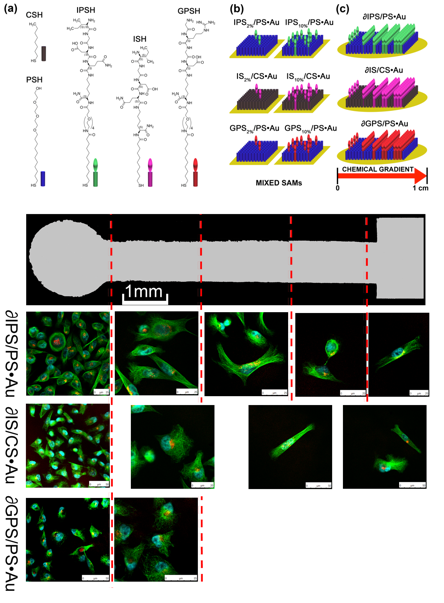
Exploiting the haptotactic cellular response and taking advantage of the SAMs approach, we have engineered motogenic surfaces in which different cells displayed different migratory reactions. Contrary to the majority of the reports in which SAMs of Arginine-Glycine-Aspartic (RGD) peptides are used to induce migratory events as a consequence of a change of the surface adhesive properties, we have used for the first time the isoleucine-glycine-aspartic (IGD) peptidic motif present in the fibronectin gelating-binding domain. By preparing mixed SAMs on Au(111) exposing a chemical gradient of IGD-containing synthetic peptide, we could prepare for the first time motogenic surfaces displaying a truly macroscopic unidirectional migratory event of the deposited cells [Small, 2016, 12, 321]. By preparing mixed SAMs on Au(111) exposing a chemical gradient of IGD-containing synthetic peptide, we could prepare for the first time motogenic surfaces displaying a truly macroscopic unidirectional migratory event of the deposited cells. In particular, comparing the migratory response of human dermal fibroblasts (isolated from fetal skin, AG04431 skin HDFs) and cancer cells (MDA-MB-231, a model of human breast cancer), a different spatio-temporal migratory response could be observed for the two cellular typologies. Propelled by these achievements, we are now expanding our studies toward more complex systems in which bidirectional (or even multidirectional) cellular movements can be remotely controlled through external stimuli. Once achieved, future perspectives in this field will tackle the supramolecular modulation and control of the interactions between cells, aiming at triggering their interconnectivity and thus intercellular communications, essential actions for ruling a collective migratory behavior. In this respect, genetically-modified models cells are currently being investigated to interface specific receptor sites with artificial recognition units that are responsive toward an external stimulus.
Focusing on the CNTs-driven activities, our team has developed the first route based on H-bond driven reversible exohedral solubilization/functionalization of multi-walled MWCNTs in apolar organic solvents [J. Am. Chem. Soc., 2011, 133, 15412]. It has been demonstrated that efficient dispersion of CNTs could be obtained through a dynamic combination of self-assembly and self-organization processes involving first the formation of a supramolecular polymer, and subsequently its intertwinement around the outer wall of the MWCNTs (multiwalled carbon nanotubes).
Aiming at extending the concept to aqueous solutions for probing biochemical properties, we have also engineered supramolecular polymers efficiently dispersing CNTs in water and in biological media, in which the lateral alkyl substituents have been replaced by PEG-type chains. This is extremely useful to further increase the water solubility of our recently designed biocompatible, magnetically-active, Fe-filled CNTs [Adv. Funct. Mater., 2013, 23, 3173; Chem. Eur. J., 2015, 21, 9288; Nanoscale, 2015, 7, 20474].
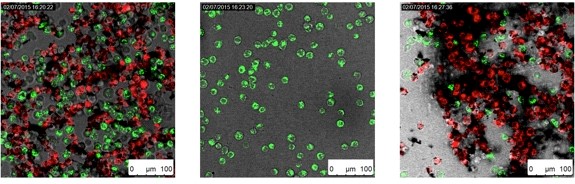
Through a covalent linkage, we recently prepared exohedrally-functionalized Fe-filled CNTs with monoclonal antibody Cetuximab (in coll. with Prof. C. Michiels, University of Namur, Belgium), known to selectively bind the epidermal growth factor receptor (EGFR), a plasma membrane receptor over-expressed (EGFR+) in several cancer cells. In-vitro magnetic filtration experiments demonstrated that a selective removal of cancer cells (red: cancer cells, green: healthy cells) from a mixed population could be obtained with the hybrid material. Aiming at understanding the nature of the interactions between the tubular framework and the antibody, molecular dynamic and docking simulations of the Cetuximab-CNT bioconjugates were performed displaying the predominant role of the hydrophobic interactions [Chem. Eur. J., 2013, 19, 12281; Chem. Soc. Rev., 2015, 44, 6916]. Experiments to determine the bio-distribution profile of the CNT hybrids through magnetic resonance imaging (MRI) both in-vivo and after organ dissection are on going.
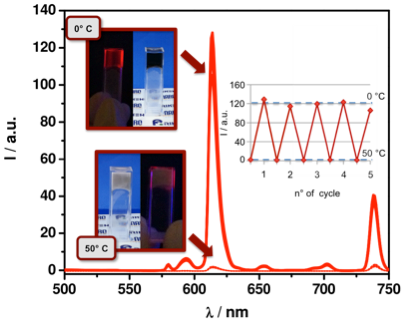
In another direction, following our seminal collaborative report with the group led by Prof. N. Armaroli (ISOF-CNR, Bologna, Italy) on luminescent CNTs [Adv. Funct. Mater., 2007, 17, 2975] in which we figured out that the low-lying electronic levels of CNTs do not quench lanthanide-centered emission, we have developed a series of protocols [Chem. Commun., 2011, 47, 1626; invited paper] for the covalent and non-covalent functionalization of MWCNTs with Eu(III) complexes. In order to further enhance the complex loading, MWCNTs were covalently functionalized with a second-generation positively-charged polycationic polyamidoamine (PAMAM) dendron presenting four ammonium groups per grafted aryl moiety [Chem. Eur. J., 2012, 18, 5889]. These newly-designed emissive CNTs hybrids are now being tested as active layers in electroluminescent devices (OLEDs, LECs) where the presence of the carbon material could provide substantial improvements of the conduction properties. In a parallel venue, we also developed an encapsulation protocol to prepare endohedral luminescent MWCNTs with a neutral and hydrophobic tris-hexafluoro acetylacetonate Eu(III) complex [Chem. Eur. J., 2011, 17, 8533]. Another encapsulated material, in which a fullerene-based carrier directed the encapsulation of a negatively-charged Eu(III) complex, has been also prepared showing an enhanced luminescent output suitable for bio-imaging applications [Nanoscale, 2014, 6, 2887].
Aiming at organizing carbon nanotubes in molecular materials, we have designed the first example of anisotropic fluorescent, thermoresponsive hydrogels containing Eu(III)-CNT hybrids, in which a water-soluble polycationic polyvinylpyridinium polymer is ion-paired with an anionic Eu(III) complex [Adv. Mater., 2013, 25, 2462]. Upon alignment of the CNT frameworks induced via a magnetic field, the emission properties of the hydrogel resulted anisotropically affected as compared to the non-magnetically treated materials. Based on these results, future directions with CNTs in our research group are focused on the design and engineering of hierarchically-structured CNTs-based materials in which organic or inorganic molecular guests are encapsulated inside the tubular hollow cavity [New J. Chem., 2014, 38, 22], exploiting in-situ and ex-situ protocols, and the external surface is exohedrally modified with covalent or non-covalent peripheral groups. While the electronic active features are dictated by the cavity-induced self-organized guest molecules, the final applications (i.e., catalysis, electrochromic or emitting devices) influence the choice of the external functionalization [Chem. Eur. J., 2015, 36, 12769]. Parts of the research objectives described in this section are supported by the European ‘SACS’ project (NMP-FP7 framework). Two main materials are targeted: a) CNT templates shaping hierarchical photosynthetic systems and b) functional platforms for magnetic-driven applications [Chem. Eur. J., 2015, 21, 9288].